INFLUENCES OF THE RELATIVE HUMIDITY ON THE STRENGTH OF GLASS BONDS EXECUTED WITH ARALDITE®2020 AND HXTAL™NYL-1
Autor:
Jelizaveta Tsedenova, Patrick Storme, Joost Caen
Year:
Anno 2015
Category:
Research
Studies in the field of conservation, concerning the strength of a bond between glass and epoxy adhesives (incl. Hxtal™NYL-1 and Araldite®2020), that have been done so far, indicate very good initial bonding strength. Keeping that in mind, occasionally interferences obstruct with a proper bond formation. As a result very low quality bonds or an unsuccessful bonds occur. This article shows the results of a research, that investigated a limited parameters in relation to glass bonding (1).
KEYWORDS: ARALDITE® 2020, EPOXY RESIN ADHESIVES, GLASS BONDING, HXTAL™ NYL-1, HUMIDITY INFLUENCE
INTRODUCTION
Glass has a fragile nature. It is a relatively hard yet brittle material and can become mechanically rather easily damaged. Hence the necessity to restore important objects made from glass like stained-glass windows, glass vessels, etc. One of the most common problems in historical glass objects are fractures. Prior to the development of synthetic bonding agents, it was a challenging task to restore the visual unity of broken pieces of glass. Fractures in stained-glass windows used to be mended by placing thin mending-lead cames to hold pieces together, vessel glass objects were sometimes reassembled with braces. Natural resins, glues (gelatine based from bone, skin, fish) and waxes were used as a bonding material (3). All these means have major disadvantages − the mechanical treatments usually interfere heavily with an overall light translucent impression of the object. The strength of the bond formed between the glass surface and the applied natural bonding materials tends to be insufficient to provide a needed support.
In the mid-20th century, technology such as the development of new synthetic materials, progressed rapidly. Due to the disadvantages of the historical glass conservation methods, conservators were eager to experiment with new synthetic materials. After more than a half of a century of experiences, there are two bonding materials that are frequently used by glass conservators worldwide. These are the epoxy resin adhesives Araldite®2020 and Hxtal™NYL-1. But as the earlier methods, also the epoxy resin adhesives might cause problems. One of the issues is that sometimes the required strength of the bonds between glass and bonding material is not achieved.
Studies in the field of conservation, concerning the strength of a bond between glass and epoxy resin adhesives (incl. Hxtal™NYL-1 and Araldite®2020), that have been done so far (4,5,6) indicate very good initial bonding strength. Keeping that in mind, occasionally interferences obstruct with a proper bond formation. As a result very low quality or unsuccessful bonds occur. The need for more specific data about possible influences on the bonding process has arisen in the glass conservation studio (Conservation Studies, Faculty of Design Sciences at the University of Antwerp). Students have reported a number of failures of epoxy-glass bonds that were done with Araldite®2020 without a presence of a detectable reason. The most concerning case occurred at the end of academic year 2010-2011. Several students executed glass bondings using Araldite®2020 within approximately a same time frame. The bonds of all the students fell apart after merely a week.
Many different factors or combinations of factors could be responsible for the epoxy-glass bond failure. The exact cause(s) are however extremely hard to determine. It was decided to investigate one parameter: relative humidity. This study will therefore be focusing on the influences of the relative humidity (RH) on the fracture repair of sheet glass bond with the epoxy resin adhesives Hxtal™NYL-1 and Araldite®2020. In the theoretical part of this paper the mechanism of bond forming between epoxy and glass is being described. The practical part sets up a series of experiments with different levels of relative humidity have any influence on the strength of the bond. The goal is to have a better understanding of the influences of the humidity on the bond. There are number of questions to be answered. The first question would be if relative humidity does intervene with polymerisation process at all? Is there any humidity needed to form a strong bond? Are there any certain humidity levels which can cause an insufficient bond between the glass and the bonding agent? In case the humidity does have a negative influence on the created bond, what are the humidity levels that most likely will cause the failure? What are the ranges of RH that are needed in order to achieve the best results?
BACKGROUND INFORMATION
According to J. Down (7) a perfectly successful synthetic glass bonding agent has many requirements and there exists yet no such material that satisfies all of them at the same time. The bond created between adhesive and glass surface must be strong enough to hold securely broken pieces together. But at the same time, it cannot be excessively strong as it would create unnecessary tension in the glass that could cause further damage to object (8). The adhesive should be removable on a request, thus making the treatment reversible and this process must not do further damage to the object. In order to create an impression of a whole object, adhesive should have an identical or very similar reflective index to glass. The adhesive should age well, meaning it has to retain its bonding strength, it should not yellow or in any way show significant deterioration evidence over certain period of time (9).
Environmental influences such as temperature, relative humidity, pollutants or other factors could have an impact on the end result of the bonding. As there might be many different reasons for the epoxy resin adhesive, that are used for conservation purpose, to fail, the studies have been done to investigate the causes. E.g. the influence of the environment such as the impact of a high temperature (40–48 oC) on epoxy resin adhesives (incl. Araldite®2020 and Hxtal™NYL-1) has been tested by Silva da Nuntes (10). The study concentrates on the temperature fluctuation but does not mention any humidity levels. A study done by Haynes looks into the humidity influences on the curing of Araldite®2020, Hxtal™NYL-1 and Fynebond® (11). Haynes is interested if high humidity levels can cause cloudiness of the epoxy infill or leave it tacky. Tests were carried out in a climate chamber with different humidity levels and constant temperature level of 20 oC. The results of her study show that both Hxtal™NYL-1 and Araldite®2020 have curing inconsistencies while being exposed to high humidity levels – in the environment of 80% RH and above some samples cure to be clouded and/or tacky. Even though the results of Haynes’ study show that high humidity might be a factor that has an impact on the epoxy resin adhesive curing efficiency, the details of the problem are still unclear.
Criteria for Bonding Material Preparation and Application in Conservation
For the optimal bond the surfaces of the parts that are to be joined have to be as clean as possible. Any grease, dirt or moisture can affect the reaction between the adhesive and the surface of the object (12). The container, where the epoxy resin adhesive will be prepared, has to be made of plastic or glass. The same material suggestion also applies to stirring and measuring object. The proportion of component A to component B has to be strictly respected. After being weighed out, the two parts have to be thoroughly stirred. Koob (13) suggests stirring for one to two minutes and after that setting the mixture aside for five minutes preferably in a warm place. After five minutes the mixture should be stirred again for at least a minute. This procedure makes the mixture homogenous thus giving it a potential to perform to its maximum. The manufacturer of Araldite®2020 mentions that the pre-treatment of a surface that will be bonded is essential to a successful bond. The surface has to be thoroughly cleaned for example with acetone. In order to achieve the strongest bonds it is suggested to mechanically abrade or chemically etch the surface. The last is never performed during conservation treatment, thus the first step has to be followed with care. After being applied, the epoxy resin adhesive has to cure. In case of Hxtal™NYL-1 most conservators agree that the curing time is seven days. Though the package information claims that it is 120 hours, meaning five days. The curing time for Araldite® 2020 seems to bring up more different opinions. The manufacturer suggests that the epoxy is fully cured after 25 hours. Authors of several different researches on epoxy resin adhesives advise that the curing time for Araldite® 2020 is 24 to 36 hours (14,15). To achieve a successful bond it is suggested that one should use epoxy resin adhesives in a moderate conditions of 20–25 oC and 40–55% RH (16), weigh the components accurately, stir thoroughly the mixture, prepare the surface by carefully cleaning it and let the epoxy resin adhesive cure for the demanded amount of time.
Surface of the Glass
Structure
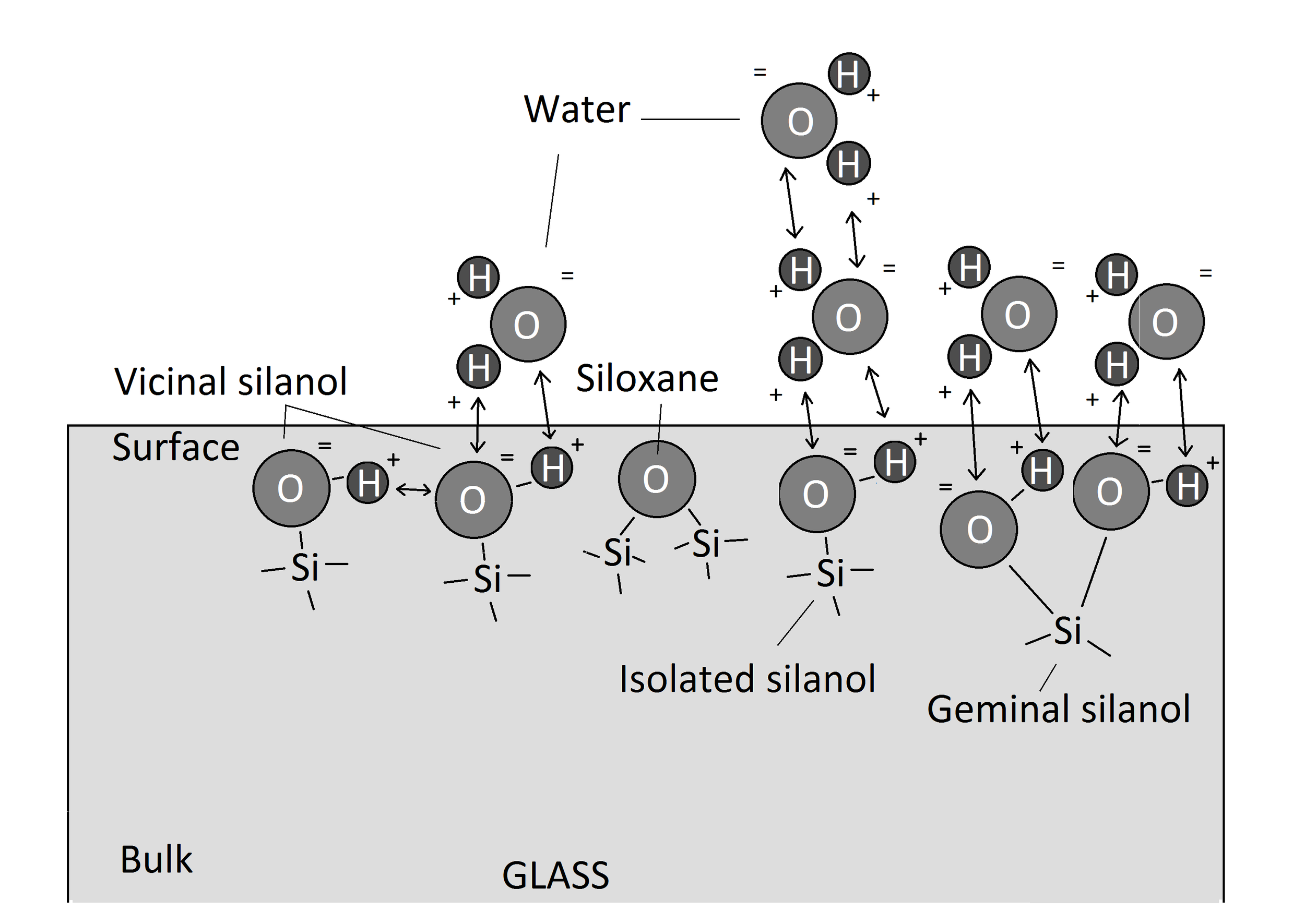
Glass and amorphous silica are hydrophilic (17). The surface of amorphous silica is different from the bulk. The bulk consists mainly of SiO2 molecules and other network modifiers such as Na2O, K2O and CaO (18). The surface of the amorphous silica consists mainly of silanols (SiOH) and some exposed siloxane bonds (Si-O-Si). There are three kinds of silanols on the surface: isolated, geminal, and vicinal [Fig. 1]. Bonded silanols can be formed of two or more molecule groups. The silanols are considered to be hydrophilic and make readily hydrogen bonds. The siloxane is usually considered hydrophobic and it can't form hydrogen bonds with H-donor adsorbents (19).
Water Monolayer Formation
Water molecules are highly polar and readily form bonds with other polarised molecules or ions (20). Air humidity consists of water molecules and if the water molecule from the air comes in contact with some other polar surface, the water molecule forms an H-bond with the surface [Fig. 1]
Fig. 1
A simplified diagram showing a different types of molecules on the surface of the glass and attraction of the water molecules. Hydrogen bonding marked with arrows.
When the surface is covered with the first water monolayer, the second, third and even more layers can be formed. Each next layer is held in place with weaker intensity. The amount of water layers that would be formed depends on the relative humidity. That is because RH is determined by two factors. These are molecule activity and - concentration. With the higher RH the molecules are slower and/or more concentrated. Thus they form bonds more easily and in greater extent (21). According to the research of Sumner et al. (22) in the low humidities there are only couple of water monolayers on the surface of the glass. Their number starts to slightly increase from a RH 50 %. The more rapid water monolayer number rise can be observed from a RH 60 %.
Adhesion
When a glass object is mechanically broken, in the place of the fracture some molecules are lost and bonds between molecules or atoms are broken (23). There are two available methods to reassemble a fractured glass object – mechanical means (as lead cames, braces etc.) and adhesives. In order to analyse why the successful bond between glass and adhesive is not achieved, one has to understand the mechanics of adhesion. To obtain an optimal quality of the bonding, it is very important to know how the adhesion process works and what factors can have a crucial influence on it.
Wetting of the Surface
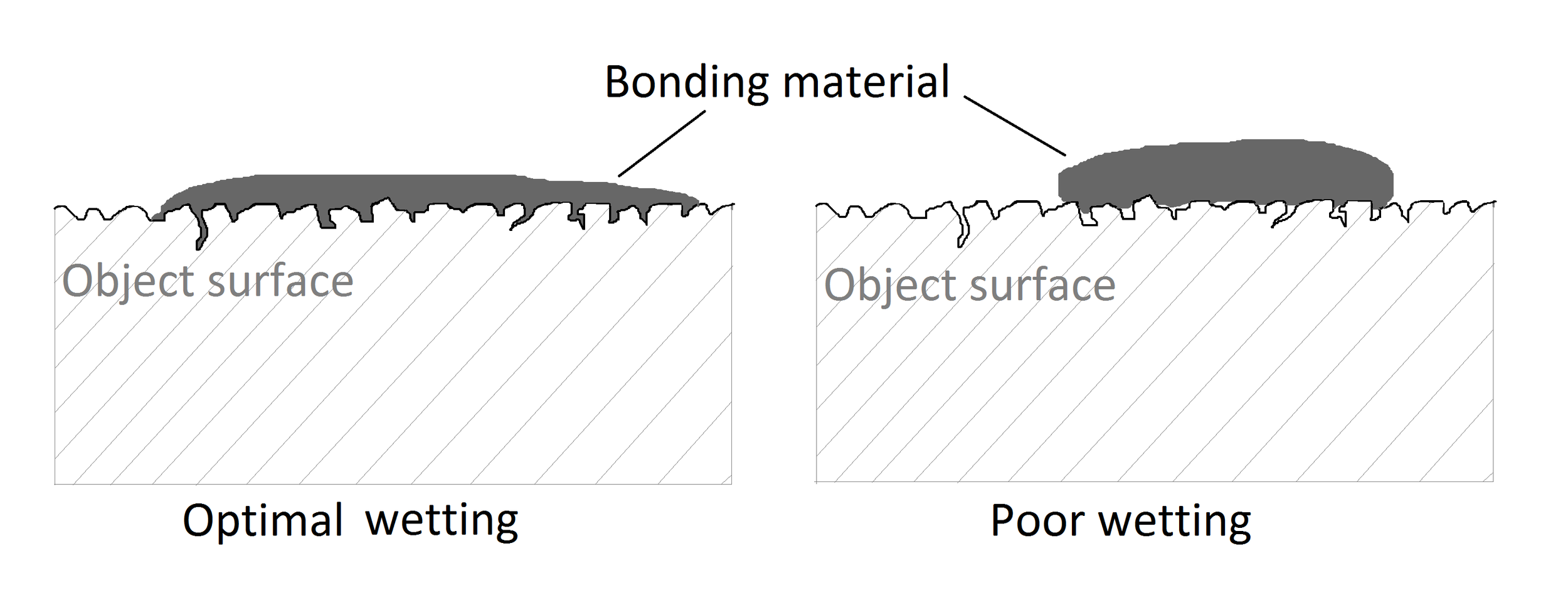
In order to have an adhesive working on its best abilities, it has to make the maximum contact with the surface. Wetting the surface means how much contact the adhesive makes with the surface. Wetting is the displacement of air or other gases present on the surface of an object by an adhesive or any other liquid. The result of good wetting is a larger contact area between the adherent and the adhesive over which the forces of adhesion may act (24). The adhesive requires a lower surface tension than the critical surface tension of the object (25). If the surface tension of the adhesive is low, the material flows readily across the surface, otherwise its molecules would be rather holding to one another than make contact with the surface. Insufficient wetting means that some of the air or foreign substance (water, grease etc.) is still trapped between adhesive and the surface (26) [Fig. 2]. As the result of poor wetting, the adhesive has smaller contact with the surface and less bonds between the object and the adhesive are formed. E.g. the monolayers of the water on the glass surface can prevent the bonding agent from spontaneously spreading on the surface, thus wetting it (27).
Adhesive Principles
The adhesive is in a significantly smaller proportions to the object, but still forms a strong enough bond to securely hold two pieces together (28). This is achieved by uniformly distributing the stresses, that occur from mechanical forces, across the joint (29). As adhesive bonding provides larger surface area for stress transfer, no particular part is overly stressed (30). The interfacial forces holding the two parts of the object together may arise from Van der Waals forces, chemical bonding or mechanical interlocking. The mechanical interlocking occurs on very rough or porous surfaces (31). When cured, adhesive is "trapped" inside the unevenness of the surface. The interlocked part of adhesive is at the same time linked to the adhesive in between of the surfaces of the object, bonding them together. Since the surface of the glass is relatively smooth, the mechanical interlocking is unlikely to be the primary joining mechanism in glass bonding. If the adhesive has a chemical functional groups that can react to the silanol groups on the glass surface, the covalent bonds will be created (32). When the adhesive has no such groups, the other way of bond formation it through hydrogen bonding and van der Waals forces (33). When a hydrogen atom H has formed a primary bond with another atom X that is more electronegative than H (34), a dipole is formed. The dipole has a positive charge at the H end (35) and therefore attractive forces may occur between H and another atom with negative charge. Bond can be formed within the same or another molecule (36). Term of van der Waals forces is sometimes used loosely for the totality of nonspecific and attractive or repulsive intermolecular forces (37). Mostly in comparison to the primary bonds, these forces are very weak. The strongest form of van der Waals forces is hydrogen bond. The term includes: dipole–dipole, dipole-induced dipole and London (instantaneous induced dipole-induced dipole) forces (38).
Bond Failure
Under a certain levels of mechanical stress adhesive bonds fail. The bonds can break due to cohesion− or adhesion failure. In case of cohesion failure the adhesive is not properly cured and the bonds inside the adhesive self break (39). The adhesive failure occurs between the surface and the adhesive. The adhesive failure is due to a weak boundary layer (40). The weak boundary layer can originate from the adhesive, the adherent or the environment (41). E.g. the adsorbed water monolayers on top of the glass surface can act as a contaminant. The adhesive forms bonds with whatever within its surrounding has greater attractive forces. Depending on the quantity of the water molecules on top of the glass, the adhesive might be drawn to form more bonds to the water molecules than the surface of the glass. Such bonds result in a weak boundary layer and if exposed to certain levels of mechanical stress, fail. Usually bonds break because of the combination of the cohesion− and adhesion failure (42).
EXPERIMENTAL
Equipment
The experiments were performed on plain 3 mm float glass. The glass was ordered from AGC Glass Europe in two 0.5 m x 1 m sheets. Sample objects were made by cutting these sheets in 2 cm x 18 cm rectangular pieces using a glass cutter. The same cutter was used to cut samples in two. To hold the freshly broken pieces together a transparent self-adhesive solvent free Tesa® tape was used. All the experiments were carried out in a custom made glove-box [Fig. 3]. The glove-box is made of 5 mm thick Plexiglass. It has the following measurements: 100 x 50 x 60 cm. There are three tightly sealable glove-holes (Ø 20 cm), one bigger hole for the equipment transfer (Ø 30 cm) and one small hole (Ø 3 cm). The smallest hole was used to install a Vaisala® Humidity and Temperature Meter HMH45. To obtain the airtight space, cardboard and tape were used. The glove-box has inside a power point with four sockets.
The temperature and the relative humidity inside the glove-box were constantly monitored by one data logger (MicroLite® II Temperature/RH 32K, serial number 9130587), starting from the third experiment there were two data loggers. The second data logger was ATAL® ATV-11 data recorder, serial number 13932477. MicroLite® II took the measurements each 10 minutes, ATAL® each 15 minutes. Due to delay in delivery the ATAL® data logger remained in the glove-box from the start of the third experiment till the end of all the experiments. To distribute the air inside the glove-box a Hama® USB Fan with Info Display was used.
Five 200 ml plastic measurement cups were installed upside down in the glove-box. One of the cups was used to support ATAL® data logger. With the help of the rest of the four plastic cups, trays that carried the samples were installed 10 cm from the bottom of the glove-box. The trays were made from 40 x 18 x 0,3 cm float glass that was covered with clear cling film. The cling film was applied for security reasons as not to cut oneself on the glass edges. Also, it prevented spilled epoxy to bond between the samples and the tray. The test samples were supported on the two 1 x 2 cm pieces of dental wax.
For the weight measurements of the epoxy components, a Sartorius® TE612 scale with 0.01 g precision was used. The epoxy resin parts were mixed in the 200 ml plastic cup. In order to transfer both components of Hxtal™NYL-1 to the mixing cup, the original containers, with very small openings, were used. For the mixture to be homogenous, the two components were stirred with one plastic pipette. Both components A and B of Araldite®2020 had to be transferred in the mixing cup using plastic pipettes. The pipette that was used to transfer component B, served as a stirring rod.
Saturated Salt Solutions
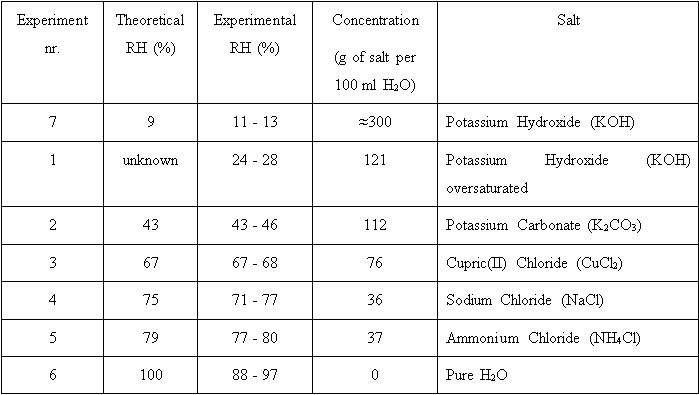
The humidity inside the glove-box was regulated using saturated salt solutions. Before the actual experiments, some tests with saturated NaCl solution were carried out in order to determine the optimal size for a contact surface. It is suggested to use the largest contact surface and the smallest vapour space possible (43). It was established that the contact surface of 289 cm2 was enough for the solution to overnight stabilize the RH in the glove-box. The solution was always placed in the same plastic container with a 289 cm2 contact surface. Salt solutions were created from chemically pure salts and distilled water (44). Accidently KOH solution was oversaturated, but it was decided to proceed with the experiment. The experience showed that oversaturated KOH solution allowed acquiring a constant higher RH than saturated KOH solution. An air circulation was needed to stabilize the relative humidity in the glove-box quicker (45) and to keep the humidity in the glove-box homogenous. There were performed seven series of experiments with seven different setups in total. [fig 4]
Protocol
Using the glass cutter, twenty pieces of sample glasses (18 x 3 x 0.3 cm) per experiment (ten for Hxtal™NYL-1 and ten for Araldite®2020) were cut in two asymmetrical parts, 5 and 13 cm. The glass was cut perpendicular to the longest side. After being cut, pieces were held together with three narrow stripes of tape (≈ 2 x 0.2 cm). Two tapes were placed on the cut side, and one on the other side. As the samples were freshly cut and had no contact with contaminants, they were not degreased. Reassembled samples were placed on two trays and provided with an information tag (date of experiment, number of experiment and the bonding material used). Each sample half was supported by a piece of dental wax [Fig 5].
Both trays were then placed inside the glove-box, on top of the supporting plastic cups. The salt solution was inserted into the glove-box, alongside with the scale, two plastic cups, one large and two small pipettes. Also a glass jar with cotton swabs and some paper towel. The paper towel was used to wipe off the excess of the bonding agent or to rest the used pipettes. The containers with bonding agents were placed in the glove-box. The glove-box was sealed and the fan was powered. The glove-box was left overnight to acquire the desired relative humidity level until the next day.
Starting the experiments in the morning, the fan was switched off, as it disturbed the scale. First the two components of Hxtal™NYL-1 were measured – 9 g of component A and 3 g of component B. The adhesive was mixed for two minutes. Next the Araldite®2020 was measured – 10 g of component A and 3 g of component B. Before mixing the small pipette was thoroughly cleaned from the component B in the paper towel, to use it for the mixing of the components. The adhesive was mixed for two minutes. Then it was set aside and Hxtal™NYL-1 was mixed again for one minute, followed by the last one minute blending of Araldite®2020. Using a fresh pipette, first one drop of the Araldite®2020 was applied on the break of each sample in its series, followed by the Hxtal™NYL-1 in its series of samples. It was observed that the epoxy was pulled into each fracture by the capillary forces. After all the fractures were filled, the cotton swab was used to remove the epoxy drop from the samples. The glove-box was opened for approximately 15 sec to take out the scale (it was needed elsewhere), paper towel (hygroscopic) and mixed epoxies (needed for other bonding). The glove-box was sealed as soon as possible. The fan was switched on again. The glove-box remained untouched for six days. The following week the samples were removed from the glove-box and the procedure was repeated with the next salt solution.
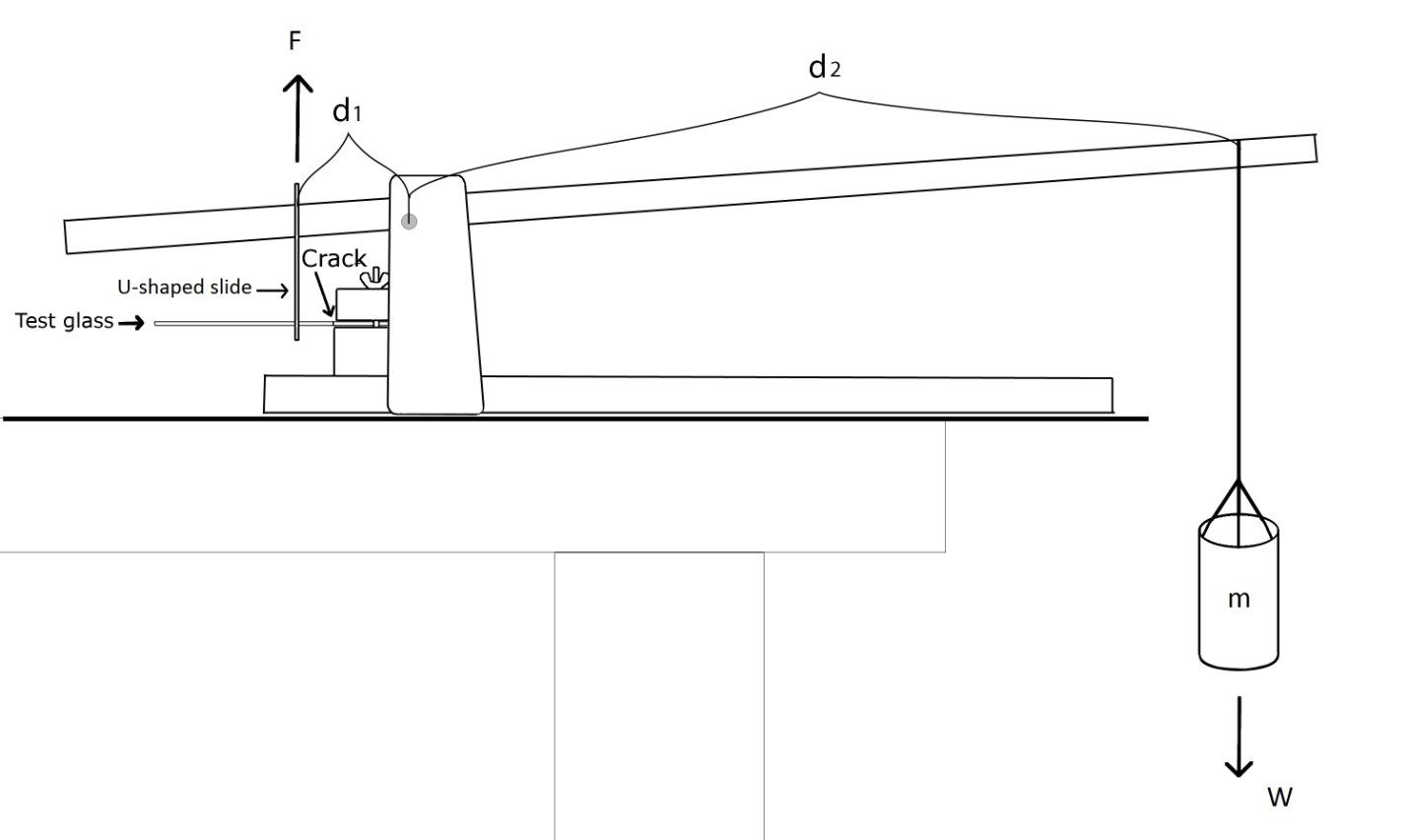
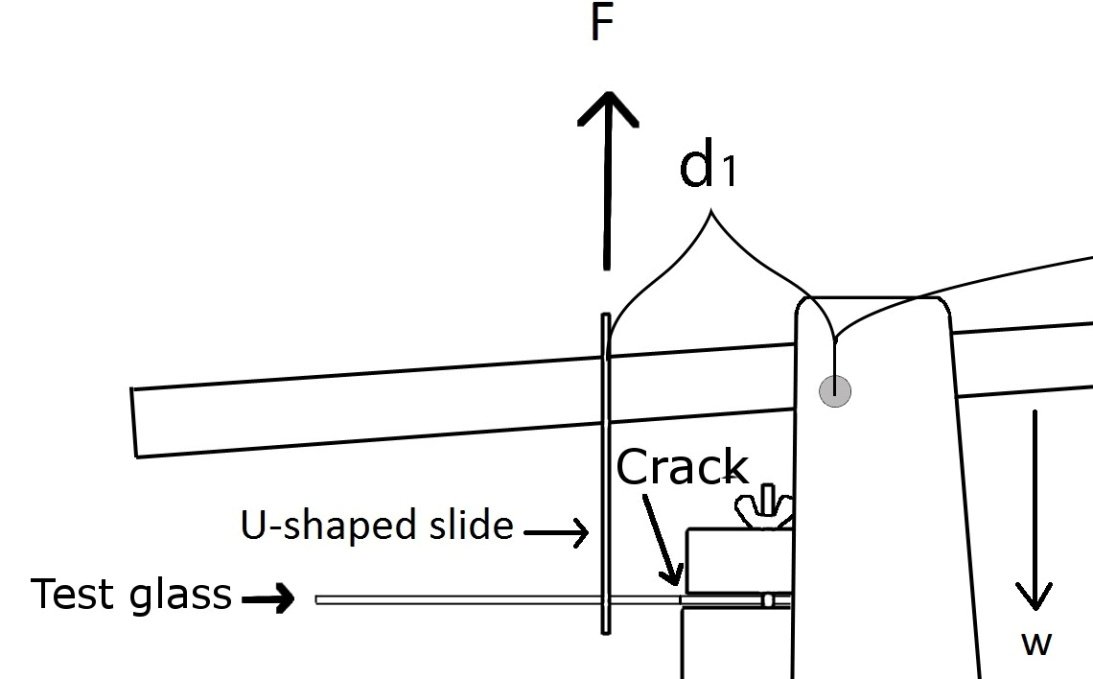
Three weeks after the each test series, the samples were taken out of the glove-box, strength tests were performed. The tapes that used to hold two pieces together were removed with a scalpel. To test the samples on their physical resistance, a breaking point testing machine was used. The bonded fracture was situated very close to the clamp [Fig. 6] and the sample glass was secured in place by tightening the screws. [fig 6] The sample glass was placed in a U-shaped metal slide that was connected to the upper part of the machine. A plastic container was placed on the other end of the machine. The container was gradually filled with rice. The compound weight of the rice and the container m created a force F that was applied by metal U-shaped slide on the sample glass. At some point in time the test glass would break in at the bonded fracture. The weight of the container at the time of the break was measured and the force that was needed to break the glass was calculated. As long as there is equilibrium the torque applied on the slide is equal but opposite to the torque of the compound weight. The torque on the compound weight is equal to its weight W x d1, is equal to the torque on the glass slide F x d2.
W (N) = m (kg) x g (m/s2)
g = 9,81 m/s2
F = 9.81 m/s2 x m (kg) x d1 (cm)/ d2(cm)
m = mass (kg) applied at the breaking of the sample glass
W = weight (N) applied at the breaking of the sample glass
F = force (N) that was needed to break the sample glass
d1 = 6 * 10-2 m , d2 = 45 * 10-2 m
The formula used to calculate the breaking force: F = m * g * 645 (m * g * 6)/45
In [Fig. 7] can be seen more closely how the sample glass was fixed in the machine. The load on the other end of the machine pressed down (marked with W on the scheme), as the sample glass was connected to the machine by U-shaped metal slide, that created a force bending it up (marked with F on the scheme).
RESULTS
Inside the tightly sealed glove-box seven separate test series with different relative humidity levels were performed. RH and temperature inside the glove-box were constantly recorded using the data logger MicroLite®II. For the sake of accuracy and comparison reasons, a second data logger ATAL® ATV-11 was used. Due to a delivery delay following the order, ATAL® ATV-11 was used starting from the third experiment.
After comparing the data from two data loggers, it was determined that the fluctuation of both RH and temperature was comparable to both devices, with the minor deviation of the ATAL® ATV-11 that showed the RH level constantly approximately 1 % lower. As ATAL® ATV-11 was recently calibrated and more accurate appliance, in this paper the RH readings from MicroLite®II will be considered 1 % too high. There were performed seven separate experiments. They will be called Test 1 for first experiment, Test 2 for second etc.
Course of the Experiments
Test 1 aimed to create the lowest RH possible using Potassium Hydroxide saturated salt solution. During the experiment it became clear that very little water is needed to saturate the KOH and the solution was accidently oversaturated. It was decided to first use oversaturated solution. The RH achieved was around 24 % and stable. Even though the glove-box was tightly sealed, during the preparation and the application of the bonding material the inner RH was influenced by the surrounding RH. By the end of the sample preparation the inner RH had raised approximately 4 %. After the treatment was completed, the RH inside the glove-box regained its previous level. The inner RH remained stable during the total time of the experiment. The similar tendency was observed in the course of every experiment − the inner RH would be influenced by the surrounding RH, would change for couple percent and regain its value after some time. Three weeks after one week curing process, the breaking point tests were performed. On all samples, the adhesive covered the whole fracture surface. During the tape removal not one of the samples demonstrated the instability. As a result there were ten different mass values for each adhesive that broke the test samples. The standard deviation was calculated to eliminate results that were too high or too low. From the results within the standard deviation range the average was calculated. This value was considered the average force that was needed to break the sample from Test 1. The test samples that were bonded in the RH 24−28 % with Araldite® 2020 required an average breaking force of 26.39 ± 7.07 N. For the Hxtal™ NYL-1 this was 36.82 ± 7.13 N. From the Test 1 it was clear that Hxtal™NYL-1 had stronger adhesive properties than Araldite® 2020, cured in the tested circumstances. The surfaces of the breaks were examined. From the observation of every experiments' surfaces could be seen, that in most of the cases the bond broke due to a combination of cohesion− and adhesion failure. The adhesive on the surface was mostly ripped perpendicularly to the surface in one or two places, leaving some portion of the adhesive on one side and some on the other side of the sample glass. In these cases the adhesion failed in greater extent than cohesion. In minority of the cases the pure adhesive failure was detected.
The goal of Test 2 was to create the "ideal" RH of around 45 %. For that purpose Potassium Carbonate was used. The RH achieved was around 43 % and stable. All of the samples for both bonding materials felt to be securely bonded. The adhesive covered the whole surface of the fracture. During the tape removal not one of the samples demonstrated any instability. The test samples that were bonded in the RH 43−46 % with Araldite® 2020 required an average breaking force of 31.56 ± 4.32 N. For the Hxtal™ NYL-1 that was 43.84 ± 5.85 N.
Test 3 was designed to produce a RH that was slightly higher than the recommended RH. For that purpose Cupric(II) Chloride was used. RH achieved was around 67 %. The inner RH recovered from a 2 % drop during the application to the previous level and raised to be approximately 1 % higher than in the beginning of the experiment. The RH stayed stable for the rest of the experiment. The adhesive covered the whole surface of the fracture. During the tape removal not one of the samples demonstrated any instability. The test samples that were bonded in the RH 67−68 % with Araldite® 2020 required an average breaking force of 19.85 ± 5.44 N. For the Hxtal™ NYL-1 that was 30.82 ± 5.76 N.
Test 4 wanted to increase a little bit the RH from Test 3 in the hope to see a tendency. In order to create a RH of around 75 % a saturated solution of Sodium Chloride was used. By the end of the sample preparation the inner RH had dropped approximately 2 %. After some time the RH had restored itself and continued raising for next three days being in the end about 4 % higher than before the sample treatment. Is case of one sample bonded with Hxtal™ NYL-1, the adhesive failed to cover about one fourth of the surface of the fracture. One of the samples bonded with Araldite® 2020 broke during the tape removal. The test samples that were bonded in the RH 73−77 % with Araldite® 2020 required an average breaking force of 16.15 ± 4.46 N. For the Hxtal™ NYL-1 that was 30.35 ± 5.76 N.
Test 5 wanted to increase the RH from Test 3 even further. In order to create a RH of around 80 % a saturated solution of Ammonium Chloride was used. By the end of the sample preparation the inner RH had dropped approximately 2 %. After some time the RH had restored itself and continued raising for next couple of days being in the end about 3 % higher than before the sample treatment. In four cases out of ten samples bonded with Araldite® 2020, the fracture was not fully covered with adhesive. The test samples that were bonded in the RH 77−80 % with Araldite® 2020 required an average breaking force of 13.57 ± 5.35 N. For the Hxtal™ NYL-1 that was 26.95 ± 9.82 N.
The purpose of Test 6 was to create the extreme maximum RH. A container with pure water was placed inside the glove-box. For the beginning of the sample treatment the inner RH was 89 %. By the end of the sample preparation the inner RH had dropped approximately 2 %. After some time the RH had restored itself and continued raising for the whole duration of the experiment, being in the end of the week 97 %. Both adhesives failed to fully cover any of the sample fractures. Samples were instable and very fragile. In case of two sampled bonded with Araldite® 2020, they broke without adding any weight to the machine. It is important to note that every time during the application it was observed very carefully that the adhesive would fully penetrate and cover the surface of the fracture. The test samples that were bonded in the RH 89−97 % with Araldite® 2020 required an average breaking force of 7.89 ± 2.97 N. For the Hxtal™ NYL-1 that was 10.47 ± 6 N.
For Test 7 it was decided to reach the other end of extreme RH conditions. The saturated Potassium Hydroxide was used to achieve RH of 13 %. It was decided to test another dry condition because the results from the first and the second experiments suggested that low RH intervenes with optimal bond forming. By the end of the sample preparation the inner RH had raised approximately 2 %. After some time the RH had restored itself and stabilised at around 11 %. The adhesive covered the whole fracture surface. During the tape removal not one of the samples demonstrated any instability. The test samples that were bonded in the RH 11−13 % with Araldite® 2020 required an average breaking force of 29.52 ± 6.41 N. For the Hxtal™ NYL-1 that was 41.4 ± 6.19 N.
ANALYSIS OF THE RESULTS
The test results show that the strongest bonds were achieved with the application and the curing at an RH of 43 %. For Araldite® 2020 the average strength was 31.52 ± 4.32 N and for Hxtal™ NYL-1 43.84 ± 5.85 N.
Results for the Low Relative Humidity
After analysing the results of the experiments, it was observed that in the low RH levels the average performance of the adhesives was slightly less efficient. In the RH 11 % the average braking force for Araldite® 2020 was 29.52 ± 6.41 N and for Hxtal™ NYL-1 41.4 ± 6.19 N. In the RH 11 % the average braking force for Araldite® 2020 was 26.39 ± 7.07 N and for Hxtal™ NYL-1 36.82 ± 7.13 N. But when the standard deviation was taken into consideration, the results did not differ as much. In case of RH 11 %, the results were almost fully falling into RH 43% standard deviation range. Similar observation could be made for the RH 24 %. Considering this analysis could be said that the bonding strength of the adhesives Araldite® 2020 and Hxtal™ NYL-1 is similar in relative humidities up to 50 %.
Results for the High Relative Humidity
By studying the graph depicted in Fig. 6 could be seen that from approximately RH 50 %, the bonding strength decreases linearly in proportion to the increase of relative humidity. That means the higher was the RH during the treatment, the weaker was the achieved bond. This observation could be applied in case of both tested epoxy adhesives. The bond was the strongest at the RH 43 % and the weakest at the RH 90 %. The fact that Araldite® 2020 lost at the RH 67 % on average a considerable amount of its initial bonding strength suggests that it is rather humidity sensitive adhesive. According to Hxtal™ NYL-1 information sheet, epoxy would cure with the same result in RH 50 % as in RH 95 % (45), but it was determined that also the bond strength executed with Hxtal™ NYL-1 decreases linearly in relation to relative humidity. Thus it could be stated that both tested epoxy resin adhesives are humidity sensitive.
Comparison of Araldite® 2020 and Hxtal™ NYL-1
In comparison between two adhesives, the Hxtal™ NYL-1 had in all the RH conditions stronger adhesive properties than the Araldite® 2020. Considering that both adhesives performed the best in the RH 43 % and their bonding strength started decrease at the higher RH levels, Hxtal™ NYL-1 demonstrated at the RH 67% and the RH 75 % almost comparable bonding strength results as the one of the Araldite® 2020 in RH 43 %.
Hypothesis for the RH Influence on the Bonding Efficiency
In order to presume if and why the RH has an influence on the bonding strength, in the beginning of the paper the adhesion mechanism and the water monolayer on the surface of the glass was described. It was noted that the strongest bonds between an object surface and an adhesive are formed in case of optimal wetting of the surface. If optimal wetting of the surface is not achieved, a weak boundary layer would form. That could cause a premature adhesive failure of an epoxy. When there are many water monolayers adsorbed to the surface of the glass, the adhesive tends to form bonds with the water molecules on the surface, thus creating less bonds with the surface itself. That kind of bonding results in a weak boundary layer. Observation of the broken sample glass surfaces showed that in the majority of the cases a combination of adhesive− and cohesive failure had occurred. Almost every time the adhesion failure could be observed in a much greater extent than the cohesion failure. From the research of Sumner et al. was determined that in the low humidities there are only couple of water monolayers on the surface of the glass. Their number starts to slightly increase from a RH 50 %. The more rapid water monolayer number rise can be observed from a RH 60 %. These numbers support the results obtained from this study. Both tested adhesives demonstrated that if handled in relative humidity up to 45 %, the optimal bonding strength was achieved. In case of the tests executed in higher RH levels, a clear decreasing linear trend of the bonding strength could be observed. The relation between executed test results and the theory suggests that the strength of the bondings made with the epoxy adhesives Araldite® 2020 and Hxtal™ NYL-1 is in a direct correlation with a relative humidity of the room where the application and the curing of the adhesives take place.
CONCLUSION
From the results of the experiments can be seen that elevated relative humidity, during the application and curing process, has a direct impact on the strength of the bond forming between glass and the adhesives Araldite® 2020 and Hxtal™ NYL-1. Bonds executed with higher RH levels proved to be weaker. When the RH reaches extreme heights, none of the two tested adhesives are able to perform to a satisfactory result. Even though the experiments indicated that Hxtal™ NYL-1 has in general a better bonding strength, it is important to note that in order to obtain the best results, both Araldite®2020 and Hxtal™NYL-1 should be handled in the working space where the RH is not higher than 50 %, since the best results for both adhesives were obtained up to RH 43 %. Values higher than 60 % RH clearly show worse results, which can be the reason for failures in the glass conservation bonding applications.
REFERENCES:
1.I would like to thank my principal supervisor Prof. Dr. Joost Caen and my second supervisor Prof. Drs. Patrick Storme for their guidance and support in writing this article.
2.Davison, S. 2009 ‘A History of Joining Glass Fragments’ in Amber, J. et al (eds.) Holding it all Together; Ancient & Modern Approaches to Joining, Repair & Consolidation, British Museum & Archetype Publications, pp. 107 -112.
3.Ibid
4.Shashoua, Y., Ling, D. A Comparison of Fynebond, Hxtal NYL-1 and Araldite 2020 Epoxy Adhesives for Use in Conservation of Glass. – Conservation News, no. 66, July 1998, p. 33–36.
5.Down, J. 1989 “Adhesive Testing at the Canadian Conservation Institute. Past and Future” Preprints to Paris Congress “Adhesives and Consolidants” 2-8 sept. pp. 18–20.
6.Haynes, J., A study of the effects of humidity on the curing of epoxy resins, Hxtal™NYL-1, Araldite®2020 and Fynebond – Adhesives and Consolidants for Ceramics and Related Materials, Transcripts of Lectures Given at the Ceramics and Class Conservation Group Meeting, UKICCGCG, 1997.
7.Down, J. 1989
8.Ibid
9.Ibid
10.Nunes da Silva, C. The heat deflection temperature of epoxy resins: A comparison of three products used in porcelain restoration, Conservation of Glass and Ceramics. In Tennent, N. (ed), Conservation of glass and ceramics: research, practice and training conference, Conservation of glass and ceramics: research, practice and training; James & James, London, 1999, pp. 132−137.
11.Haynes, J. 1997
12.Davison, Down, Ebnesajjad, Koob, Petrie, both manufacturers of Araldite®2020 and Hxtal NYL-1™.
13.Koob, S. P., Tips and tricks with epoxy and other casting and molding materials – AIC Objects Specialty Group Postprints, Volume Ten, 2003, pp. 158–165.
14.Shashoua, 1998.
15.Haynes, J. 1997.
16.Ibid.
17.Davison, S., Conservation and Restoration of Glass, Second Edition, Elsevier Ltd., Oxford, 2003, pp. 266.
18.Vogel, W., Glass Chemistry, Second Edition, Springer-Verlag, Berlin, 1994, pp. 123.
19.Nawrocki, J., Mickievicz, A., The Silanole Group and its Role in Liquid Chromatography, in Journal of Chromatography A, 779, 1997, pp. 29−71.
20.Ed.-in-chief Wheatcroft, A., Series Ed. Wilks, H., Science for Conservators: Conservation Science Teaching Series, Heritage Series Heritage: Care, Preservation, Management. Museums and Galleries Commission. Conservation Unit Volume 2 of Science for Conservators, Cleaning, Routledge, Oxon, 1992, pp. 75.
21.Camuffo, D., Fernicola, V., Havermans, J. (Editors), How to Measure Temperature and Relative Humidity. Instruments and Instrumental Problems. In Basic Environmental Mechanisms Afecting Cultural Heritage. Understanding Deterioration Mechanisms for Conservation Purposes. COST Action D 42: CHEMICAL INTERACTIONS BETWEEN CUTURAL ARTEFACTS AND INDOOR ENVIRONMENT (ENVIART), Nardini Editore, Florence, 2010, pp. 31–42.
22.Sumner, A. L., Menke, E., Dubowski, Y., Newberg, J., Penner, R., Hemminger, J., Wingen, L., Brauners, T. And Finlayson-Pitts, B. The Nature of Water on Surfaces of Laboratory Systems and Implications for Heterogenous Chemistry in the Troposphere, Physical Chemistry Chemical Physics, No. 6, 2004, pp. 604−613.
23.Ed.-in-chief Wheatcroft, A., Series Ed. Wilks, H., Science for Conservators: Conservation Science Teaching Series, Heritage Series Heritage: Care, Preservation, Management. Museums and Galleries Commission. Conservation Unit Volume 3 of Science for Conservators, Adhesives, Routledge, Oxon, 1992, pp. XX.
24.Ebnesajjad, pp. 5.
25.Ibid, pp. 7.
26.Petrie, pp. 56.
27.Davison, 2003, pp. 206.
28.Ed. Ebnesajjad, S., Handbook of Adhesives and Surface Preparation. Technology, Application and Manufactoring, Elsevier Ltd., Oxford, 2011, pp. 3.
29.Wu , S., Polymer Interface and Adhesion, Marcel Dekker, New York, 1982, pp. 337.
30.Ebnesajjad, 2011, pp 3.
31.Habenicht, G., Applied Adhesive Bonding: A Practical Guide for Flawless Results, Wiley-VCH, Weinheim, 2009, pp. 57.
32.Plueddemann, E., Silane Coupling Agents, Second Edition, Springer Science, New York, 1991, pp. 18.
33.Petrie, E., Handbook of Adhesives and Sealants, McGrow-Hill, New York, 2000, pp. 50.
34.Arunan, E., Desiraju, G. R., Klein, R. A., Sadlej, J., Scheiner, S., Alkorta, I., Clary, D. C., Crabtree, R. H., Dannenberg, J. J., Hobza, P., Kjaergaard, H. G., Legon, A. C., Mennucci, B., Nesbitt, D. J. Definition of the hydrogen bond, Pure and Applied Chemistry, Vol 83, No. 8, 2011, pp. 1637−1641.
35.Jeffrey, G. A., Saenger, W., Hydrogen Bonding in Biological Structures, Springer-Verlag, Berlin, 1994, pp. 15.
36.Arunan, 2011.
37.IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford,1997, pp. 1588.
38.Parsegian, A., Van Der Waals Forces. A Handbook for Biologists, Chemists, Engineers and Physicists, Cambridge University Press, New York, 2006, pp. 6.
39.Ebnesajjad, 2011, pp. 10.
40.Bikerman, J. J., The Science of Adhesive Joints, Academic Press Inc., New York, 1968, pp. 2.
41.Ebnesajjad, 2011, pp. 5.
42.Ibid, pp. 12.
43.Hygrodynamics, Inc., Creating and maintaining humidities by salt solutions. Liggett & Myers, Maryland, 1996.
44.Greenspan, L., Humidity Fixed Points of Binary Saturated Aqueous Solutions, in Journal of Research of the National Bureau of Standarts - A. Physics and Chemistry, Vol. 81A, No. 1, 1977, pp. 89−96.
45.Hygrodynamics, Inc., 1996.
46.Hxtal NYL-1 Information sheet, His Glassworks, Inc.
BIBLIOGRAPHY
Arunan, E., Desiraju, G. R., Klein, R. A., Sadlej, J., Scheiner, S., Alkorta, I., Clary, D. C., Crabtree, R. H., Dannenberg, J. J., Hobza, P., Kjaergaard, H. G., Legon, A. C., Mennucci, B., Nesbitt, D. J. Definition of the hydrogen bond, Pure and Applied Chemistry, Vol 83, No. 8, 2011, pp. 1637−1641.
Bikerman, J. J., The Science of Adhesive Joints, Academic Press Inc., New York, 1968.
Camuffo, D., Fernicola, V., Havermans, J. (Editors), How to Measure Temperature and Relative Humidity. Instruments and Instrumental Problems. In Basic Environmental Mechanisms Afecting Cultural Heritage. Understanding Deterioration Mechanisms for Conservation Purposes. COST Action D 42: CHEMICAL INTERACTIONS BETWEEN CUTURAL ARTEFACTS AND INDOOR ENVIRONMENT (ENVIART), Nardini Editore, Florence, 2010.
Davison, S., Conservation and Restoration of Glass, Second Edition, Elsevier Ltd., Oxford, 2003.
Davison, S. ‘A History of Joining Glass Fragments’ in Amber, J. et al (eds.) Holding it all Together; Ancient & Modern Approaches to Joining, Repair & Consolidation, British Museum & Archetype Publications, 2009.
Down, J. “Adhesive Testing at the Canadian Conservation Institute. Past and Future” Preprints to Paris Congress “Adhesives and Consolidants” 2-8 sept. 1989.
Ed. Ebnesajjad, S., Handbook of Adhesives and Surface Preparation. Technology, Application and Manufactoring, Elsevier Ltd., Oxford, 2011.
Greenspan, L., Humidity Fixed Points of Binary Saturated Aqueous Solutions, in Journal of Research of the National Bureau of Standarts - A. Physics and Chemistry, Vol. 81A, No. 1, 1977.
Haynes, J., A study of the effects of humidity on the curing of epoxy resins, Hxtal™NYL-1, Araldite®2020 and Fynebond – Adhesives and Consolidants for Ceramics and Related Materials, Transcripts of Lectures Given at the Ceramics and Class Conservation Group Meeting, UKICCGCG, 1997.
Hygrodynamics, Inc., Creating and maintaining humidities by salt solutions. Liggett & Myers, Maryland, 1996.
Jeffrey, G. A., Saenger, W., Hydrogen Bonding in Biological Structures, Springer-Verlag, Berlin, 1994.
Koob, S. P., Tips and tricks with epoxy and other casting and molding materials – AIC Objects Specialty Group Postprints, Volume Ten, 2003.
Compiled by McNaught, A. D. and Wilkinson, A., IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Blackwell Scientific Publications, Oxford, 1997.
Nawrocki, J., Mickievicz, A., The Silanole Group and its Role in Liquid Chromatography, in Journal of Chromatography A, 779, 1997.
Nunes da Silva, C. The heat deflection temperature of epoxy resins: A comparison of three products used in porcelain restoration, Conservation of Glass and Ceramics. In Tennent, N. (ed), Conservation of glass and ceramics: research, practice and training conference, Conservation of glass and ceramics: research, practice and training; James & James, London, 1999.
Parsegian, A., Van Der Waals Forces. A Handbook for Biologists, Chemists, Engineers and Physicists, Cambridge University Press, New York, 2006.
Petrie, E., Handbook of Adhesives and Sealants, McGrow-Hill, New York, 2000.
Plueddemann, E., Silane Coupling Agents, Second Edition, Springer Science, New York, 1991.
Shashoua, Y., Ling, D. A Comparison of Fynebond, Hxtal NYL-1 and Araldite 2020 Epoxy Adhesives for Use in Conservation of Glass. – Conservation News, no. 66, July 1998.
Sumner, A. L., Menke, E., Dubowski, Y., Newberg, J., Penner, R., Hemminger, J., Wingen, L., Brauners, T. And Finlayson-Pitts, B. The Nature of Water on Surfaces of Laboratory Systems and Implications for Heterogenous Chemistry in the Troposphere, Physical Chemistry Chemical Physics, No. 6, 2004.
Vogel, W., Glass Chemistry, Second Edition, Springer-Verlag, Berlin, 1994.
Ed.-in-chief Wheatcroft, A., Series Ed. Wilks, H., Science for Conservators: Conservation Science Teaching Series, Heritage Series Heritage: Care, Preservation, Management. Museums and Galleries Commission. Conservation Unit Volume 2 of Science for Conservators, Cleaning, Routledge, Oxon, 1992.
Ed.-in-chief Wheatcroft, A., Series Ed. Wilks, H., Science for Conservators: Conservation Science Teaching Series, Heritage Series Heritage: Care, Preservation, Management. Museums and Galleries Commission. Conservation Unit Volume 3 of Science for Conservators, Adhesives, Routledge, Oxon, 1992.
Wu, S., Polymer Interface and Adhesion, Marcel Dekker, New York, 1982.